This clinical trial aims to evaluate the efficacy and safety of a novel hearing aid technology developed by the Hearing Care Center in Fort Worth, Texas. The study seeks to assess the impact of the innovative device on improving hearing outcomes and enhancing the quality of life for individuals with hearing loss by hearing care center.
Study Design:
- Study Type: Randomized controlled trial
- Participants: Adults aged 50 years and older with mild to moderate sensorineural hearing loss residing in Fort Worth and surrounding areas.
- Sample Size: 100 participants (50 intervention group, 50 control group)
- Duration: 6 months
Study Objectives:
To assess the effectiveness of the novel hearing aid technology in improving speech recognition and auditory perception in individuals with mild to moderate hearing loss.
To evaluate the impact of the hearing aid technology on participants’ quality of life, communication abilities, and overall satisfaction with auditory outcomes.
To determine the safety profile of the novel hearing aid technology, including any adverse events or discomfort experienced by participants.
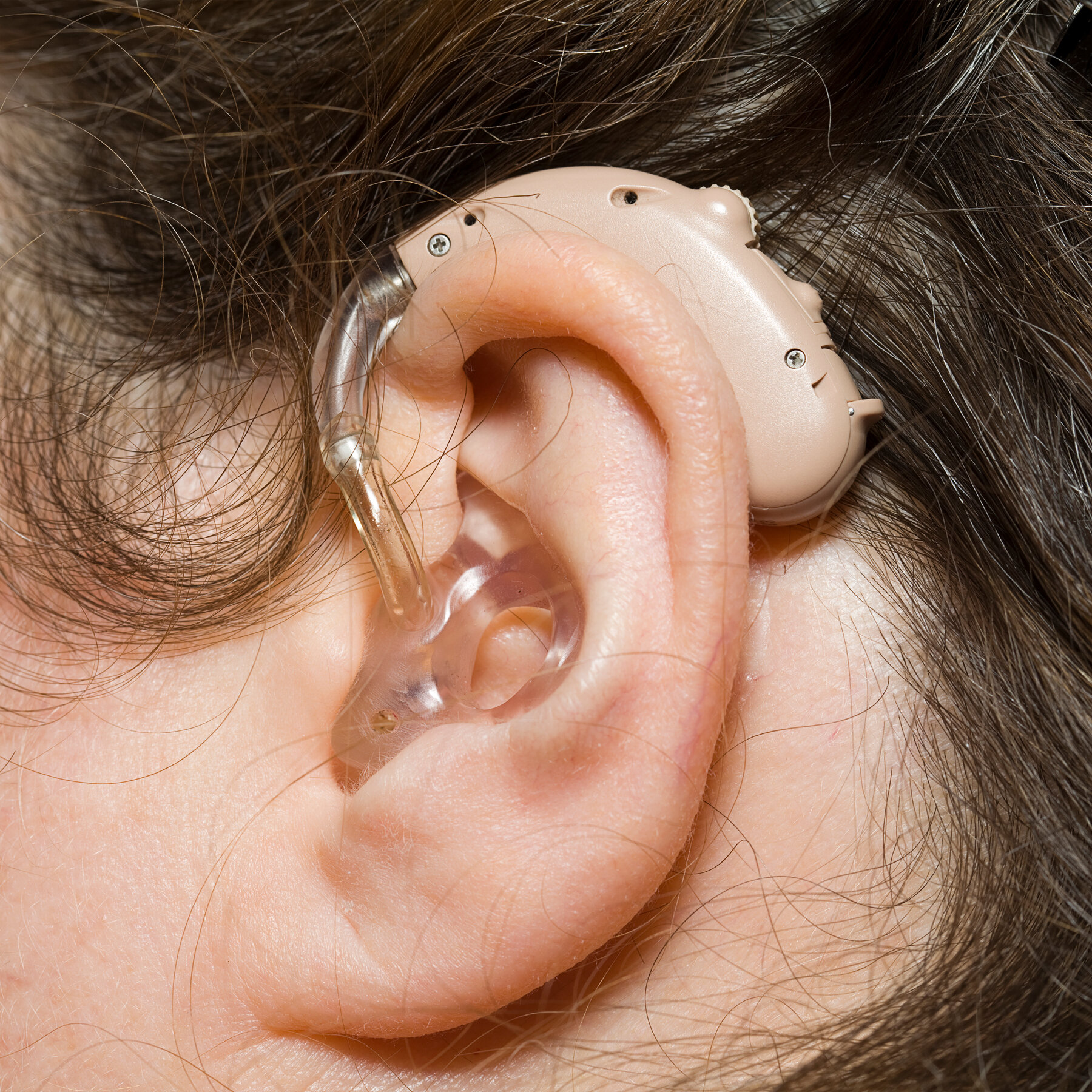
Study Procedures:
- Participant Screening: Eligible individuals will undergo screening assessments, including audiometric testing and medical history review, to confirm eligibility for the study.
- Randomization: Participants will be randomly assigned to either the intervention group (receiving the novel hearing aid technology) or the control group (receiving standard hearing aid treatment).
- Intervention: Participants in the intervention group will be fitted with the novel hearing aid technology and provided with personalized programming and adjustment sessions by experienced audiologists from the Hearing Care Center.
- Outcome Measures: Participants will undergo baseline assessments and follow-up evaluations at 3- and 6-months post-intervention to measure changes in hearing outcomes, communication abilities, and quality of life.
- Data Analysis: Statistical analysis will be conducted to compare outcomes between the intervention and control groups, including speech recognition scores, self-reported measures of satisfaction, and adverse events.
Expected Outcomes:
It is anticipated that participants in the intervention group will demonstrate significant improvements in speech recognition, auditory perception, and quality of life compared to those in the control group.
The novel hearing aid technology is expected to be well-tolerated with minimal adverse effects, indicating its safety and suitability for individuals with hearing loss.
Conclusion:
This clinical trial conducted in hearing care center holds the potential to contribute valuable insights into the efficacy and safety of a novel hearing aid technology in improving auditory outcomes and enhancing the quality of life for individuals with hearing loss. The findings of this study may inform future advancements in hearing healthcare and guide the development of innovative interventions to address the needs of individuals with hearing impairment.
